- Clinical Study
- Romosozumab in Postmenopausal Korean Women with Osteoporosis: A Randomized, Double-Blind, Placebo-Controlled Efficacy and Safety Study
-
Ki-Hyun Baek, Yoon-Sok Chung, Jung-Min Koh, In Joo Kim, Kyoung Min Kim, Yong-Ki Min, Ki Deok Park, Rajani Dinavahi, Judy Maddox, Wenjing Yang, Sooa Kim, Sang Jin Lee, Hyungjin Cho, Sung-Kil Lim
-
Endocrinol Metab. 2021;36(1):60-69. Published online February 24, 2021
-
DOI: https://doi.org/10.3803/EnM.2020.848
-
-
6,855
View
-
390
Download
-
7
Web of Science
-
10
Crossref
-
 Abstract Abstract
 PDF PDF Supplementary Material Supplementary Material PubReader PubReader  ePub ePub
- Background
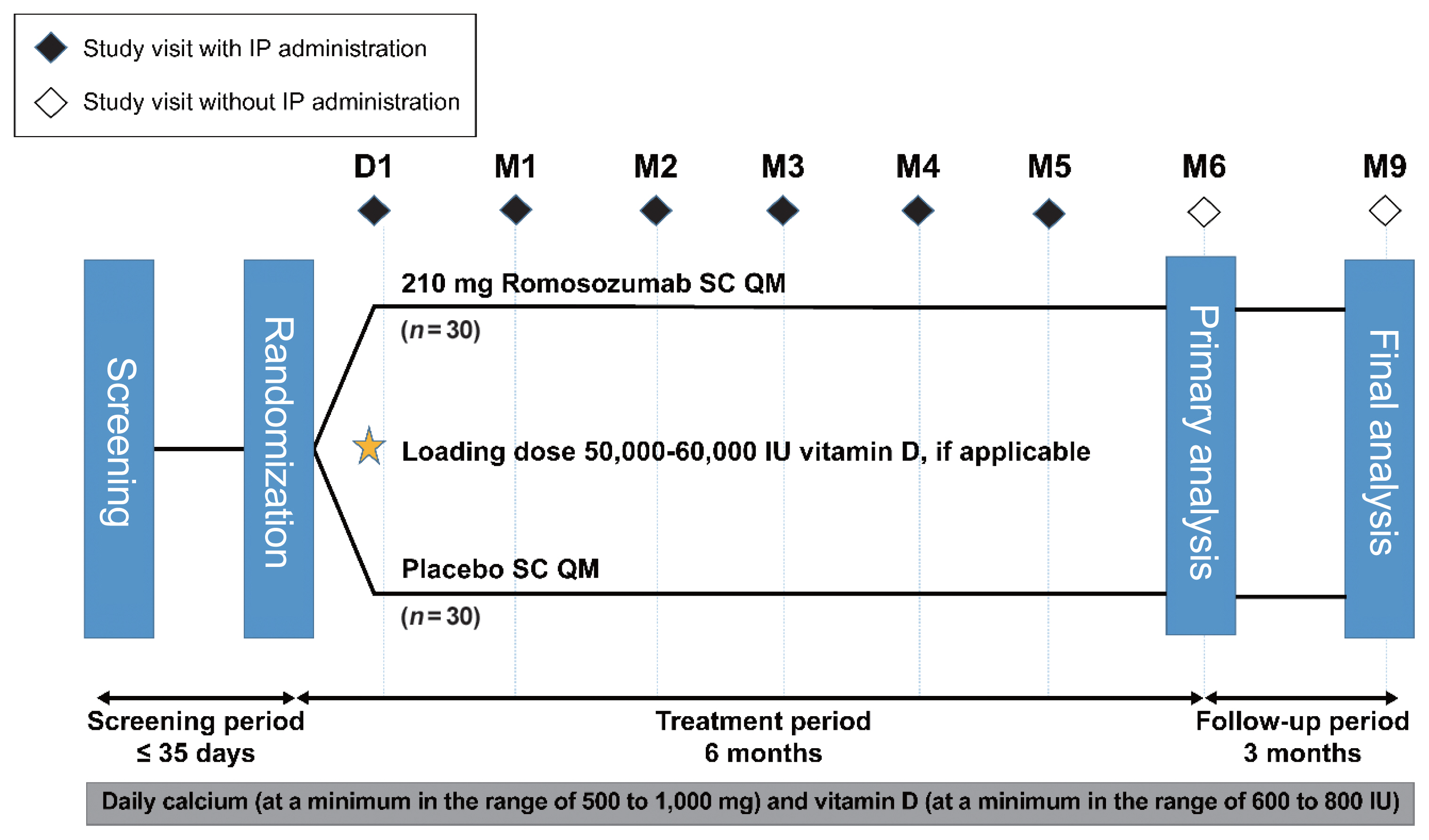
This phase 3 study evaluated the efficacy and safety of 6-month treatment with romosozumab in Korean postmenopausal women with osteoporosis.
Methods
Sixty-seven postmenopausal women with osteoporosis (bone mineral density [BMD] T-scores ≤–2.5 at the lumbar spine, total hip, or femoral neck) were randomized (1:1) to receive monthly subcutaneous injections of romosozumab (210 mg; n=34) or placebo (n=33) for 6 months.
Results
At month 6, the difference in the least square (LS) mean percent change from baseline in lumbar spine BMD (primary efficacy endpoint) between the romosozumab (9.5%) and placebo (–0.1%) groups was significant (9.6%; 95% confidence interval, 7.6 to 11.5; P<0.001). The difference in the LS mean percent change from baseline was also significant for total hip and femoral neck BMD (secondary efficacy endpoints). After treatment with romosozumab, the percent change from baseline in procollagen type 1 N-terminal propeptide transiently increased at months 1 and 3, while that in C-terminal telopeptide of type 1 collagen showed a sustained decrease. No events of cancer, hypocalcemia, injection site reaction, positively adjudicated atypical femoral fracture or osteonecrosis of the jaw, or positively adjudicated serious cardiovascular adverse events were observed. At month 9, 17.6% and 2.9% of patients in the romosozumab group developed binding and neutralizing antibodies, respectively.
Conclusion
Treatment with romosozumab for 6 months was well tolerated and significantly increased lumbar spine, total hip, and femoral neck BMD compared with placebo in Korean postmenopausal women with osteoporosis (ClinicalTrials.gov identifier NCT02791516).
-
Citations
Citations to this article as recorded by  - A pharmacovigilance analysis of FDA adverse event reporting system events for romosozumab
Zepeng Chen, Ming Li, Shuzhen Li, Yuxi Li, Junyan Wu, Kaifeng Qiu, Xiaoxia Yu, Lin Huang, Guanghui Chen
Expert Opinion on Drug Safety.2023; 22(4): 339. CrossRef - Evaluation of the efficacy and safety of romosozumab (evenity) for the treatment of osteoporotic vertebral compression fracture in postmenopausal women: A systematic review and meta‐analysis of randomized controlled trials (CDM‐J)
Wenbo Huang, Masashi Nagao, Naohiro Yonemoto, Sen Guo, Takeshi Tanigawa, Yuji Nishizaki
Pharmacoepidemiology and Drug Safety.2023; 32(6): 671. CrossRef - Efficacy and Cardiovascular Safety of Romosozumab: A Meta-analysis and Systematic Review
Seo-Yong Choi, Jeong-Min Kim, Sang-Hyeon Oh, Seunghyun Cheon, Jee-Eun Chung
Korean Journal of Clinical Pharmacy.2023; 33(2): 128. CrossRef - Clinical Studies On Romosozumab: An Alternative For Individuals With A High Risk Of Osteoporotic Fractures: A Current Concepts Review (Part I)
E. Carlos Rodriguez-Merchan, Alonso Moreno-Garcia, Hortensia De la Corte-Rodriguez
SurgiColl.2023;[Epub] CrossRef - Romosozumab in osteoporosis: yesterday, today and tomorrow
Dong Wu, Lei Li, Zhun Wen, Guangbin Wang
Journal of Translational Medicine.2023;[Epub] CrossRef - Efficacy and safety of anti-sclerostin antibodies in the treatment of osteoporosis: A meta-analysis and systematic review
Frideriki Poutoglidou, Efthimios Samoladas, Nikolaos Raikos, Dimitrios Kouvelas
Journal of Clinical Densitometry.2022; 25(3): 401. CrossRef - Benefits of lumican on human bone health: clinical evidence using bone marrow aspirates
Yun Sun Lee, So Jeong Park, Jin Young Lee, Eunah Choi, Beom-Jun Kim
The Korean Journal of Internal Medicine.2022; 37(4): 821. CrossRef - What is the risk of cardiovascular events in osteoporotic patients treated with romosozumab?
I. R. Reid
Expert Opinion on Drug Safety.2022; 21(12): 1441. CrossRef - Proxied Therapeutic Inhibition on Wnt Signaling Antagonists and Risk of Cardiovascular Diseases: Multi-Omics Analyses
Yu Qian, Cheng-Da Yuan, Saber Khederzadeh, Ming-Yu Han, Hai-Xia Liu, Mo-Chang Qiu, Jian-Hua Gao, Wei-Lin Wang, Yun-Piao Hou, Guo-Bo Chen, Ke-Qi Liu, Lin Xu, David Karasik, Shu-Yang Xie, Hou-Feng Zheng
SSRN Electronic Journal .2022;[Epub] CrossRef - Multi-Omics Analyses Identify Pleiotropy and Causality Between Circulating Sclerostin and Atrial Fibrillation
Yu Qian, Peng-Lin Guan, Saber Khederzadeh, Ke-Qi Liu, Cheng-Da Yuan, Ming-Yu Han, Hai-Xia Liu, Mo-Chang Qiu, Jian-Hua Gao, Wei-Lin Wang, Yun-Piao Hou, Guo-Bo Chen, Lin Xu, David Karasik, Shu-Yang Xie, sheng zhifeng, Hou-Feng Zheng
SSRN Electronic Journal .2022;[Epub] CrossRef
|